Table of Contents
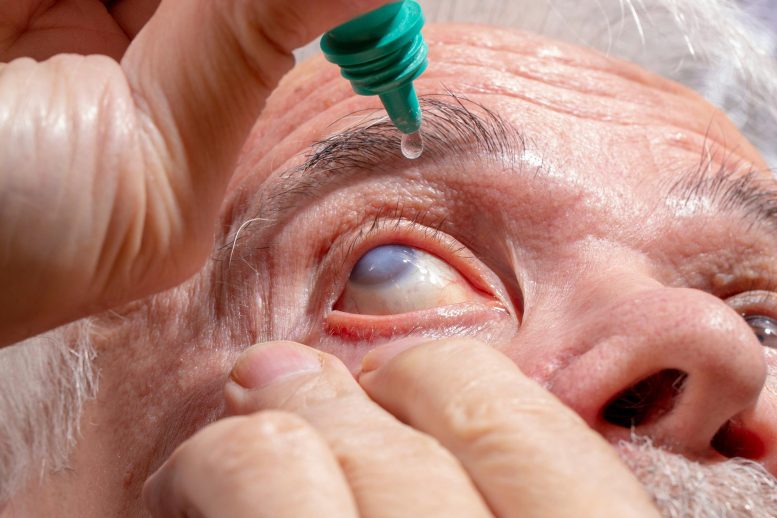
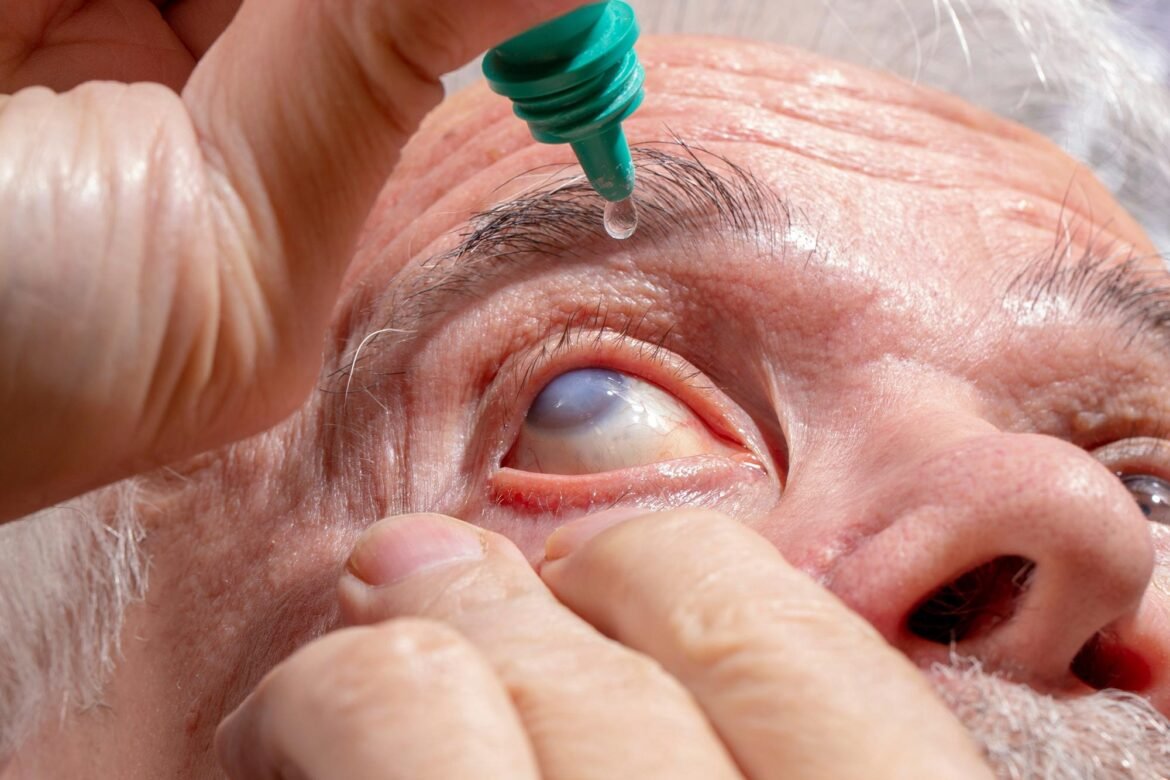
A common eye ointment may quietly damage glaucoma implants—sometimes to the point of rupture—by making them swell from the inside.
New research from Nagoya University in Japan suggests that widely used eye ointments may pose a serious risk for some glaucoma patients. The study shows for the first time, using both patient data and laboratory testing, that petrolatum-based eye ointments can damage the PRESERFLO MicroShunt, a glaucoma implant used in more than 60 countries.
The findings raise concerns about a routine eye care product that may unintentionally weaken or even rupture a device designed to protect vision.
Understanding Glaucoma and How It Is Treated
Glaucoma is a condition that damages the optic nerve and can eventually lead to vision loss. It is often caused by increased pressure inside the eye when fluid does not drain properly. Researchers estimate that glaucoma affects about 76 million people worldwide.
One modern treatment option is the MicroShunt, a tiny filtration device surgically placed in the eye to help fluid drain more effectively. Compared with traditional glaucoma surgeries, the MicroShunt generally leads to fewer complications and reduces the need for additional medications after surgery.
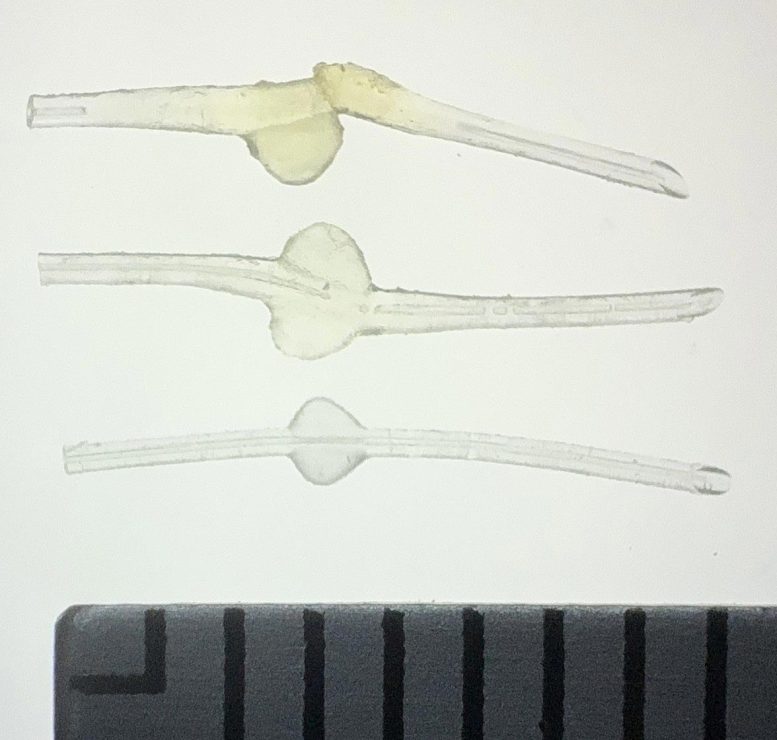
Top: MicroShunt explanted from a patient, exhibiting diffuse swelling with fracture and loss of one fin.
Middle: MicroShunt explanted from another patient, showing localized swelling around the fin.
Bottom: Unused MicroShunt (control).
Scale: 1 division = 1 mm.
Credit: Ryo Tomita
Why the MicroShunt Is Vulnerable to Certain Ointments
The MicroShunt is made from a specialized styrenic thermoplastic elastomer derived from a polystyrene-block-polyisobutylene-block-polystyrene (SIBS) block polymer. This material is valued for its flexibility, biocompatibility, and low risk of triggering inflammation or scarring.
However, the same properties that make the material effective inside the eye also make it susceptible to oil-based substances. Because the polymer has a strong attraction to oils, petrolatum-based eye ointments can seep into the implant. When this happens, the device may swell and its shape and flexibility can change.
The manufacturer specifically warns against this risk. According to official instructions, “the MicroShunt should not be subjected to direct contact with petrolatum-based (i.e., petroleum jelly) materials, such as ointments and dispersions.” Despite this guidance, the warning is not always widely known or followed in everyday clinical care.
Surgeons Observe Implant Rupture in Real Cases
“Swollen MicroShunts can be structurally fragile,” said ophthalmologist and Assistant Professor Ryo Tomita of Nagoya University Graduate School of Medicine, the study’s first author. “During surgery, I observed a rupture in a swollen MicroShunt. If more clinicians are aware of this risk, they will be able to prevent similar problems.”
To better understand what was happening, Tomita worked with Assistant Professor Taiga Inooka and Associate Professor Kenya Yuki from Nagoya University Hospital and the Graduate School of Medicine. They partnered with Dr. Takato Kajita and Junior Associate Professor Atsushi Noro from the Graduate School of Engineering to investigate how petrolatum-based ointments affect the MicroShunt.
The medical team reviewed patient cases, while the engineering team performed detailed laboratory tests. Their findings were published in Graefe’s Archive for Clinical and Experimental Ophthalmology.

Clinical Evidence From Patient Cases
The clinical portion of the study focused on seven glaucoma patients whose MicroShunt implants were later removed for various reasons. Clear differences emerged based on whether the implant had contact with ointment.
In three cases, the MicroShunt was exposed outside the conjunctiva and patients were treated with a petrolatum-based eye ointment. All three devices showed noticeable swelling, and two of them had ruptured.
In another three cases, the implant remained fully covered by the conjunctiva and no ointment was used. These MicroShunts showed no structural changes.
One additional case proved especially important. Although the MicroShunt was exposed outside the conjunctiva, no ointment was applied. The device did not swell. This strongly suggests that direct contact with the ointment, not exposure alone, is the main cause of swelling.
Laboratory Tests Confirm the Cause
Laboratory experiments supported the clinical observations. Researchers placed unused MicroShunts into petrolatum-based eye ointment to replicate what was seen in patients.
Under microscopic analysis, the changes were clear. After just 24 hours, the outer diameter of the MicroShunt increased to 1.44 times its original size. The fin-like section of the device expanded to 1.29 times its original width.
Chemical testing revealed why this happened. After 24 hours of immersion, oil components accounted for about 45% of the MicroShunt’s total weight. After three months, that figure rose to 73%.
These results confirmed that swelling occurs because oil-based ointment ingredients are absorbed directly into the implant material.
What This Means for Glaucoma Care
The researchers urge clinicians to avoid using petrolatum-based eye ointments in patients with MicroShunt implants, especially when the device is exposed outside the conjunctiva. They recommend considering alternative treatments after surgery and stress the need for further studies to determine whether swelling affects implant performance even when rupture does not occur.
“Our study found that commonly used medical materials can cause unexpected complications if their chemical properties and usage environments are not fully understood,” Noro stated. “From both medical and engineering perspectives, we emphasize the importance of understanding the chemical properties of medical materials and appropriately managing their usage environments.”
Reference: “Petrolatum-based ointment application induces swelling of the PRESERFLO MicroShunt” by Ryo Tomita, Taiga Inooka, Takato Kajita, Hideyuki Shimizu, Ayana Suzumura, Jun Takeuchi, Tsuyoshi Matsuno, Hidekazu Inami, Koji M. Nishiguchi, Atsushi Noro, and Kenya Yuki. (2026), 13 January 2026, Graefe’s Archive for Clinical and Experimental Ophthalmology.
DOI: 10.1007/s00417-025-07075-2
Funding information:
This study was supported by Grant-in-Aid for Young Scientists (21K16870) from JSPS KAKENHI; Suda Memorial Glaucoma research grant; the Japan Glaucoma Society Research Project Support Program, and Eno Science Foundation research grant.
Never miss a breakthrough: Join the SciTechDaily newsletter.
Follow us on Google and Google News.